Supplemental Oxygen for the General Aviation Pilot
At a recent West Valley Flying Club mountain flying seminar, one of the participants wondered if density altitude affected the requirement for supplemental oxygen. At first glance the answer to the question appears relatively straightforward: the FAR's are based upon cabin pressure altitude, not density altitude. But, does the method of determining "altitude" really make a difference? Just how do altitude, oxygen concentration, and the pilot and passenger's physiology modify the need for oxygen? How much of a safety margin (if any) is factored into the FAR's?
The considerations in determining the need for supplemental oxygen may be divided into four broad categories: (1) what is legal, (2) what are the considerations that affect the amount of available atmospheric oxygen, (3) what are the physiologic considerations that influence oxygen requirements, and finally (4) how much oxygen is necessary for the safety of the pilots and passengers. In attempting to address each of these four categories, the discussion in this article will be limited to non-pressurized aircraft.
What is legal?
The relevant FAR that defines what is legal reads:91.211 Supplemental Oxygen
(a) General. No person may operate a civil aircraft of U.S. registry -
(1) At cabin pressure altitudes above 12,500 feet (MSL) up to and including 14,000 feet (MSL) unless the required minimum flight crew is provided with and uses supplemental oxygen for that part of the flight at those altitudes that is of more than 30 minutes duration;
(2) At cabin pressure altitudes above 14,000 feet (MSL) unless the required minimum flight crew is provided with and uses supplemental oxygen during the entire flight time at those altitudes; and
(3) At cabin pressure altitudes above 15,000 feet (MSL) unless each occupant of the aircraft is provided with supplemental oxygen.
Atmospheric Considerations affecting Oxygen Requirements
The relative composition of the earth's atmosphere (excluding water vapor) is remarkably constant from sea level to approximately 300,000 feet (Table 1).| Gas | Composition of Dry Air (%) |
|---|---|
| Nitrogen | 78.09 |
| Oxygen | 20.95 |
| Argon | 0.93 |
| Carbon Dioxide | 0.03 |
| All Others | < 0.03 |
The mass (amount) of the gases contained in a given volume of air is directly related to the amount of pressure (compression) applied. As oxygen is a gas in the air, the mass of oxygen in a given volume of air is affected by atmospheric pressure. At a higher atmospheric pressure (lower altitude) there is more oxygen in a given volume (eg. in a given breath) than at low atmospheric pressure (higher altitude). The amount of oxygen in a given volume of air is usually measured as the partial pressure of oxygen in the mixture.
The law of physics known as Dalton's law may be used to determine the partial pressure exerted by oxygen. Dalton's law states that the pressure exerted by a mixture of gases is equal to the sum of the pressures that each gas would exert if it alone occupied the space filled by the mixture.
Pt = P1 + P2 + P3 +... where Pt = total pressure of system. P1, P1, P3, ... are partial pressures of each component. A restatement of Dalton's law is that the partial pressure of a gas in a mixture is:
Px = Fx × Pt
where Px = partial pressure of gas x, Fx = fractional concentration of gas x, Pt = total pressure of system.
For sea level (at standard pressure), this means that the ambient partial pressure of oxygen (PO2) can be calculated to be:
PO2 = (fractional concentration of O2 in atmosphere) × (atmospheric pressure)
= (20.95 / 100) × 760 mm Hg
= 159.2 mm Hg
Thus, the ambient oxygen partial pressure at sea level for comparison with other altitudes is about 159 mm Hg. Doing a similar series of calculations using Dalton's law, the ambient oxygen pressure at any atmospheric pressure can be calculated for dry air (Table 2).
| Altitude | Atmospheric Pressure (mm Hg) | Ambient O2 (mm Hg) |
|---|---|---|
| 0 | 760 | 159 |
| 5,000 | 632 | 133 |
| 10,000 | 523 | 110 |
| 12,000 | 483 | 101 |
| 13,000 | 465 | 97 |
| 14,000 | 447 | 94 |
| 15,000 | 429 | 90 |
| 20,000 | 350 | 73 |
| 25,000 | 282 | 59 |
As we breathe in air, it is rapidly modified by our nasal passages, mouth, throat and upper airways. The air is quickly warmed to body temperature (37°C) and the air is saturated with water vapor. The partial pressure of water vapor that air can hold is a function only of temperature with the vapor pressure of water equal to 47 mm Hg at 37°C. Our mucous membranes are always kept moist and therefore the supply of water "available" to be vaporized exceeds the amount required to saturate the air. Thus the saturation vapor pressure of water is constant at 47 mm Hg in the lungs regardless of altitude. (The saturation vapor pressure of water is, however, higher if we have a fever.)
Note: Atmospheric pressure equals 47 mm Hg at a pressure altitude of 63,000 feet. This means that above a pressure altitude of 63,000 feet our body fluids will boil as our body water tries to saturate the atmosphere to a partial pressure of 47 mm Hg!
The vapor pressure of the water in the lungs reduces the amount of atmospheric pressure represented by dry air in the lungs and thus the partial pressure of oxygen. For practical purposes, the variation in the amount of water vapor in the ambient air is not important in determining the need for supplemental oxygen since the water vapor pressure in our lungs is a constant. In aviation meteorological terms, at normal body temperature the dew point of the air in our lungs is 37°C. Since the dew point of ambient air is very rarely as high as 37°C, our nose, throat, and airways essentially always add water vapor to the air we breath until the constant, 47 mm Hg vapor pressure, saturation point is reached regardless of altitude.
The magnitude of the effect of water vapor in decreasing the partial pressure of oxygen in our lungs is modest at low altitude, but increases disproportionately at higher altitudes.
At sea level:
Atmospheric pressure at pressure altitude of 0 = 760 mm Hg
Vapor pressure of water at 37°C = 47 mm Hg
Fraction of inspired dry air that is oxygen = 0.21
Thus, the dry air exerts a pressure of:
760mmHg - 47mmHg = 713 mm Hg
The partial pressure of oxygen in air saturated with water vapor at 37°C is:
713 mmHg × 0.21 = 150 mmHg.
This compares with an oxygen pressure at sea level in dry air of 159 mm Hg. Thus, correcting for water vapor results in a 6% reduction in the partial pressure of oxygen.
Doing the same calculations for a pressure altitude of 14,000 feet (atmospheric pressure of 447 mm Hg) at 37°C with saturated water vapor gives a partial pressure of oxygen of 84 mm Hg compared with 94 mm Hg in dry air (an 11% reduction in oxygen partial pressure due to water vapor).
At 14,000 feet:
(447 mm Hg - 47 mm Hg) × 0.21 = 84 mm Hg)
Other Atmospheric Considerations, including Density Altitude
Density altitude is pressure altitude corrected for temperature. Since the air in the lungs is always (for practical purposes) at 37°C, the air in our lungs is always at the same density altitude for a given pressure altitude regardless of ambient temperature!
In the gas exchange portion of the lung (the alveoli), the oxygen partial pressure also falls as oxygen is absorbed into the blood and carbon dioxide is released from the blood into the alveolar air spaces. The addition of carbon dioxide to the air in the lungs creates a partial pressure for carbon dioxide that results in a reduction in the partial pressure of oxygen.
As is evident from the above information, actual alveolar oxygen is related to atmospheric pressure, the fractional percentage of oxygen in the atmosphere (constant to 300,000 feet pressure altitude), body temperature (37°C), vapor pressure of water (constant at a given temperature), the amount of carbon dioxide released (reflects workload of the body), the rate and volume of respiration, and the amount of oxygen carried away from the lungs by the blood. Table 3 gives the results of experimental measurements of actual alveolar oxygen and carbon dioxide in healthy, resting humans at the given pressure altitudes. Of all of the factors affecting the partial pressure of oxygen, only 2 are easily modified (1) atmospheric pressure (ie. cabin pressure) through changes in aircraft altitude or through cabin pressurization and (2) increasing the fractional percentage of oxygen in the inspired air.
| Pressure Altitude (ft) | Atmospheric Pressure (mm Hg) | Ambient O2 (mm Hg) | Alveolar O2 (mm Hg) | Alveolar CO2 (mm Hg) |
|---|---|---|---|---|
| 0 | 760 | 159 | 103 | 40 |
| 5,000 | 632 | 133 | 81 | 37 |
| 10,000 | 523 | 110 | 61 | 35 |
| 12,000 | 483 | 101 | 54 | 34 |
| 13,000 | 465 | 97 | 51 | 33 |
| 14,000 | 447 | 94 | 48 | 33 |
| 15,000 | 429 | 90 | 45 | 32 |
| 20,000 | 350 | 73 | 34 | 29 |
| 25,000 | 282 | 59 | 30 | 27 |
From Holmstrom, FMG: Hypoxia. In. Aerospace Medicine. Edited by HW Randall. Baltimore. The Williams & Wilkins Co., 1971
Physiologic Factors affecting Oxygen Requirements
Besides being affected by the alveolar partial pressure of oxygen, the amount of oxygen available to the tissues of the body is related to the rate of oxygen exchange between the air sacs of the lung (alveoli) and blood, the ability of the blood to carry oxygen, the rate at which the blood is circulated and the distribution of the blood among the tissues of the body.Ventilation/perfusion. In the lung, the exchange of oxygen from the air into the blood involves an intricate balancing of the amount of ventilation and the amount of blood flowing to the different regions of the lung. If air flows to a part of the lung where there is no blood flow, or blood flows to a part of the lung where there is no air flow, no oxygen exchange will occur. When the amount of ventilation and the amount of blood are optimal or near optimal (normal), it is referred to as matched ventilation/perfusion. If one area of the lung receives more or less ventilation relative to the flow of blood, it is referred to as a ventilation/perfusion mismatch. Normal matching is a dynamic process. When we are upright gravity causes more blood to flow towards the bases of our lungs, and so the lungs adjust so that most of the ventilation also goes to the bases of our lungs. When we lie down on our backs, gravity causes more blood to flow to the backs of our lungs, and the ventilation is also adjusted.
Anything that compromises the ability of our lungs to adjust ventilation for changes in blood flow and visa versa results in a mismatch. As one would expect, mismatching significantly interferes with the uptake of oxygen into the blood. Anything that causes a serious ventilation/perfusion mismatch reduces the oxygenation of blood. Common causes are lung disease such as bronchitis or emphysema (eg. from smoking or cystic fibrosis), blood clots to the lung (pulmonary emboli), pneumonia, and heart disease.
Diffusion capacity. The movement of oxygen from the air sacs of the lung into the blood is by simple diffusion. The ability of oxygen to diffuse from the air sacs into the blood is referred to as the diffusion capacity. Meaningful alterations in the diffusion capacity are relatively uncommon, but diffusion capacity is reduced with age and emphysema, and increased with exercise and the supine posture.
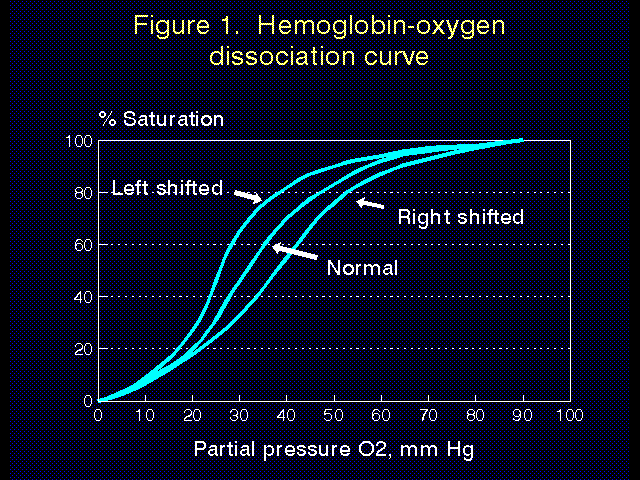
Hemoglobin and factors affecting hemoglobin. Almost all of the oxygen carried by blood is carried bound to hemoglobin (Hgb). The theoretic oxygen carrying capacity of hemoglobin is 1.39 ml O2 per gram of Hgb. A very small amount of oxygen is carried unbound (dissolved) in blood (Table 4). Each molecule of Hgb can carry 4 molecules of oxygen. The ability of Hgb to bind and hold the 4 molecules of oxygen can be expressed by the hemoglobin-oxygen dissociation curve. The higher the partial pressure of oxygen, the greater the percentage of Hgb that is carrying a full complement of 4 oxygen molecules (eg. fully saturated). The shape of the normal hemoglobin-oxygen dissociation curve is shown in Figure 1.
| Pressure Altitude (ft) | Alveolar partial pressure of O2 (mm Hg) | Dissolved O2 (ml O2/100 ml blood) | Hemoglobin Saturation (%) | O2 carried by Hemoglobin (ml O2/100 ml blood) | Total O2 in blood (ml O2/100 ml blood) |
|---|---|---|---|---|---|
| 0 | 103 | 0.32 | 97 | 20.22 | 20.54 |
| 5,000 | 81 | 0.25 | 94 | 19.60 | 19.85 |
| 10,000 | 61 | 0.19 | 90 | 18.77 | 18.96 |
| 12,000 | 54 | 0.17 | 87 | 18.14 | 18.31 |
| 13,000 | 51 | 0.16 | 84 | 17.51 | 17.67 |
| 14,000 | 48 | 0.15 | 82 | 17.10 | 17.25 |
| 15,000 | 45 | 0.14 | 80 | 16.68 | 16.82 |
| 20,000 | 34 | 0.11 | 67 | 13.97 | 14.08 |
| 30,000 | 30 | 0.09 | 57 | 11.88 | 11.97 |
T = 37°C, 1.39 ml O2 per gram of saturated hemoglobin (actual physiologic probably approximates 1.31).
Several factors are known to alter (shift) the hemoglobin-oxygen dissociation curve (Figure 1). Shifts to the left are caused by a high pH, low temperature, and high 2,3-diphosphoglycerate levels (see below). A left shift helps to increase the uptake of oxygen (good in the lungs at high altitude), but to inhibit the release of oxygen in the tissues (bad). Shifts to the right are caused by low pH, high temperature, and low 2,3-diphosphoglycerate levels. This helps to inhibit the uptake of oxygen in the lungs (bad at high altitude), but increases the release of oxygen in the tissues. As the pH is usually low and temperature high in tissues requiring oxygen, the delivery of oxygen to the tissues is positively affected by normal, physiologic changes in the blood as it circulates through the body.
2,3-diphosphoglycerate (2,3-DPG) is a molecule that also shifts the hemoglobin-oxygen dissociation curve. Concentrations of 2,3-DPG increase over several days as we acclimate to high altitude, and decrease on return to lower elevations. The role of 2,3-DPG on the physiologic adaptation to altitude is controversial. It is clear, however, that at very high altitude increased concentrations of 2,3-DPG become counter productive because they result in inhibition of oxygen release in oxygen deprived tissues.
Cardiac output. In response to reduced oxygen in the blood, the cardiovascular system responds by increasing heart rate, increasing the percentage of blood ejected from the heart with each heart beat, and by reducing the peripheral resistance of the vasculature. The net result is an increase in the amount of blood circulated through the lungs and body per minute (cardiac output). Increases in cardiac output help to compensate for the reduction in the amount of oxygen in the blood at high altitude.
Normal ventilatory drive. Increased rates of ventilation may help to overcome the affects of reduced partial pressures of oxygen. The ability to compensate by increased ventilation is, however, modest. Further, under normal conditions, the body's primary ventilatory drive is based upon the carbon dioxide concentration in the blood and not the oxygen content.
The usual "set point" that the respiratory centers strive to maintain is an arterial CO2 pressure of 40 mm Hg. With intentional hyperventilation, the CO2 content of arterial blood may be lowered to 10-20 mm Hg (the oxygen content of the blood is little affected), and the respiratory drive is diminished. This is why one can hold one's breath longer after hyperventilating than without hyperventilating. In fact, the respiratory drive from hyperventilation is inhibited enough that serious oxygen deprivation may result from breath holding following hyperventilation.
Note: This explains why it is not a good idea to hyperventilate prior to swimming underwater. You might pass out from oxygen deprivation before the respiratory drive becomes strong enough from elevated CO2 to cause you to surface and breath.
The oxygen content of the blood does influence a relatively weak, secondary respiratory drive. Increases in ventilatory drive secondary to hypoxia (low oxygen) start at approximately 4,000 feet, are modest up to an altitude of approximately 8,000 feet, and are maximal at approximately 22,000 feet. The major increase in ventilation is through deeper breaths, rather than an increase in the rate of breathing. The increased ventilation, however, results in lowering of CO2 causing an alkalosis. This alkalosis actually inhibits respiration, so that the increased ventilatory drive from hypoxia is partially counteracted.
Abnormal Physiology
A number of factors may affect the oxygen requirements of the individual. These factors include reduced hemoglobin (anemia), lung disease, age, heart disease, carbon monoxide poisoning, and smoking.Anemia. Since most of the oxygen in the blood is carried by hemoglobin, anything that reduces the concentration of hemoglobin will reduce the oxygen carrying capacity of the blood. At sea level, individuals are normally able to compensate to accommodate hemoglobin concentrations as low as 10 gm/dl (normal female 11.7-15.7 gm/dl; normal male 13.5-17.7 gm/dl). Below 10 gm/dl most individuals are symptomatic even at sea level. Individuals with anemia will benefit from maintaining partial pressures of oxygen that assure near 100% saturation of hemoglobin at all pressure altitudes.
Chronic lung disease. Individuals with most types of chronic lung disease will have impairment of ventilation or diffusion so that the oxygen content of the blood is relatively decreased. The decreased oxygen content of the blood places these individuals at increased risk of hypoxia at any altitude relative to normal individuals.
It is crucial, however, that supplemental oxygen by used extremely judiciously by individuals with chronic lung disease. With severe lung disease, the respiratory centers loose their responsiveness to CO2 concentrations, CO2 is retained, and oxygen becomes the primary driving force for respiration. As the respiratory centers drive respiration only when low partial pressures of oxygen are detected, the oxygen content of the blood is maintained at a low level (usually less than 60 mm Hg; 90% saturated hemoglobin). In individuals who retain CO2, the normal response to the inspiration of supplemental oxygen is to stop breathing (and sometimes permanently)! You are obviously looking for trouble if you fly at any altitude with an individual with serious lung disease, but should never fly with them at an altitude that requires supplemental oxygen without consulting with a physician.
Age. The normal amount of ventilation/perfusion mismatch in the lungs increases with age, so that older individuals will benefit from supplemental oxygen at lower altitude than will younger individuals.
Heart disease. As one of the normal methods of physiological compensation for low oxygen content in the blood is an increase in cardiac output, any heart disease that reduces that ability of the heart to compensate through increased cardiac output will result in an increased oxygen requirement. Again, if in any doubt, check with your passenger's physician.
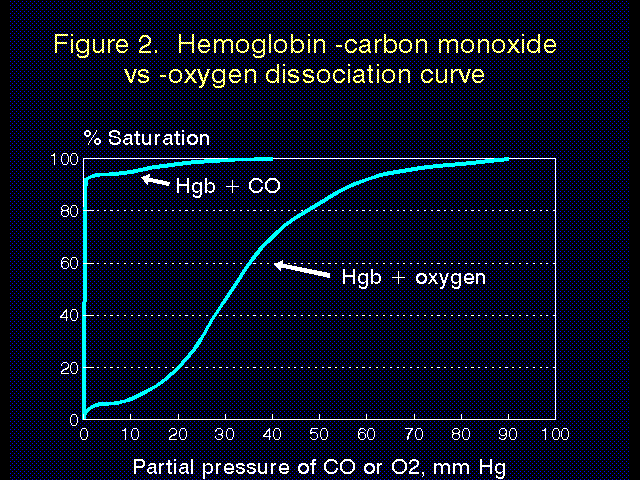
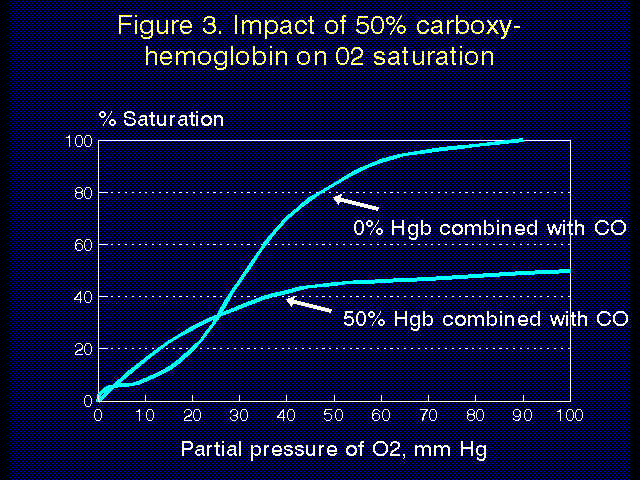
Carbon monoxide poisoning. Carbon monoxide (CO) has 300 times higher affinity for hemoglobin than does oxygen, and hemoglobin that is bound to carbon monoxide (carboxyhemoglobin) cannot bind oxygen. The high affinity of CO for hemoglobin is demonstrated in Figure 2. Hemoglobin is more than 50% saturated with CO at a partial pressure of less than 1 mm Hg. The affects of this on the oxygen-hemoglobin dissociation curve are demonstrated in Figure 3. Obviously CO is to be avoided at all costs.
Tobacco smoking. Besides placing themselves at a very high risk of chronic lung disease, cancer, and heart disease and subjecting their skin to early aging, individuals who smoke are poisoning themselves with carbon monoxide. Typical carboxyhemoglobin concentrations for smokers are 2 - 12%. With carboxyhemoglobin concentrations this high, smokers will be affected by hypoxia at lower pressure altitudes and require supplemental oxygen at lower pressure altitudes than individuals who do not smoke. (Personally, I would avoid any CFI or CFII who smokes, they clearly have no judgement!)
Alcohol. Alcohol interferes with the tissues' ability to utilize oxygen resulting in hypoxia at lower pressure altitudes than without alcohol. As one should never, ever, drink and fly, enough is said.
Other factors. Physical exertion, cold ambient temperature, concurrent illness, and many medications (including over-the-counter antihistamines) exacerbate the effects of hypoxia by either increasing the oxygen demand or interfering with the utilization of oxygen by the tissues.
Affects of Hypoxia
The onset of hypoxia is usually insidious, and most individuals will not recognize that they are experiencing hypoxia. It is therefore crucial that you train yourself to avoid hypoxia, rather than depending upon yourself to recognize the affects of hypoxia as they occur. For the normal individual, the affects of hypoxia are not detectable below pressure altitudes of 10,000 feet. Above 10,000 feet, the deleterious affects of hypoxia increase slowly at first, but then rapidly as altitude is increased above approximately 12,000 feet.Complex eye-hand coordination such as is required to maintain airspeed, heading, or vertical velocity decreases approximately 10% at 12,000 feet and 20-30% at 15,000 feet. At 12,000 feet, the dark-adapted eye begins to experience significant deterioration in vision. Unconsciousness may occur at altitudes as low as 16,000 feet in some individuals. The most dangerous aspect of hypoxia is that the individual experiencing hypoxia does not and cannot detect the decrement in function and loses the ability for critical judgement.
Any of the affects of hypoxia will occur at lower pressure altitudes in individuals with anemia, lung disease, cardiac disease, advanced age, and in individuals who smoke.
What is Safe?
The guidelines for the use of supplemental oxygen outline in FAR 91.211 are appropriate for the usual pilot who does not smoke, is in good health, and flies during day time. Smokers, individuals with any of the health problems described above, or pilots who are flying at night should strongly consider oxygen supplementation at lower pressure altitudes. The risks of hypoxia increase gradually with altitude, not step-wise as might be expected from the language of the FARs. The pilot who flies at 12,500 feet for 2 hours believing that the difference between 12,500 and 12,510 feet eliminates the risks of hypoxia is flying into trouble.Don't hesitate to use supplemental oxygen at altitudes and times not required by the FAA, especially at night, when critical judgement and hand-eye coordination is necessary (eg. IFR), or if you smoke or are not perfectly healthy. When in doubt, consider using oxygen above 10,000 feet. Think ahead of your altitude. The most dangerous aspect of hypoxia is that the individual experiencing hypoxia does not and cannot detect the decrement in function and loses the ability for critical judgement.
